Hey everyone! Ever imagined a world where we could literally print new body parts or custom tissues right when we need them? It sounds like something straight out of a sci-fi movie, but trust me, bioprinting is rapidly turning that dream into a tangible reality.
I’ve been diving deep into the latest advancements, and the progress is absolutely mind-blowing. We’re talking about a technology that’s already reshaping how we approach medicine, from drug testing to personalized treatments.
From what I’ve personally seen and felt, bioprinting isn’t just about creating rudimentary structures anymore; it’s about engineering incredibly complex tissues that truly mimic our body’s own amazing architecture.
Imagine custom-made skin grafts tailored perfectly for burn victims, or groundbreaking personalized organ models enabling far more accurate drug testing than ever before, moving us beyond archaic animal trials.
The seamless integration of AI is proving to be a monumental game-changer, refining precision and making the entire process smarter, faster, and remarkably more efficient.
Seriously, it feels like every single day brings a new, jaw-dropping breakthrough, pushing the absolute boundaries of what we thought was possible. While there are definitely significant hurdles we’re still navigating, especially in scaling up production and achieving widespread clinical translation, the sheer momentum in this field is undeniable.
This isn’t just abstract future tech; it’s actively unfolding right now, and its practical applications are getting closer to radically improving countless lives.
Let’s dive deeper and truly explore this revolutionary field together!
Crafting Custom Body Parts: The Reality of Bioprinting
Okay, so picture this: instead of waiting ages for an organ donor, or facing the terrifying possibility of your body rejecting a transplanted organ, imagine a future where a medical team could simply print you a new one, perfectly matched to your own unique biology. That’s the exhilarating promise of bioprinting, and it’s not just some far-off fantasy anymore. I’ve been completely captivated by how quickly this field is evolving. We’re talking about a process where living cells and clever biomaterials, often called bio-inks, are precisely laid down layer by layer to create intricate, functional tissues and even organs. This isn’t your average 3D printer making plastic trinkets; this is about engineering life itself, with unprecedented accuracy. The ability to use medical imaging data to create patient-specific anatomical models and then literally tailor organs or tissues for individual patients is truly revolutionary. It feels like every update I read brings us closer to a world where “personalized medicine” takes on a whole new meaning. We’re moving away from a one-size-fits-all approach to healthcare towards treatments specifically designed for you, right down to the cellular level.
The Building Blocks: How Bioprinting Works Its Magic
So, how does this actually happen? Well, at its core, bioprinting involves a few key steps. First, scientists create a digital blueprint, often from patient scans, of the tissue or organ they want to replicate. Then, specialized bioprinters meticulously deposit bio-inks—these are basically biocompatible materials loaded with living cells, growth factors, and other biological components—in precise 3D patterns. It’s like an inkjet printer, but instead of ink, it’s using living cells to build structures. The goal is to mimic the natural extracellular matrix of native tissues, providing the cells with the perfect environment to grow, differentiate, and organize into functional tissue structures. The control over scaffold architecture, composition, pore shape, and size is absolutely crucial here, allowing researchers to create incredibly complex tissue tailored to specific needs. This level of precision is something traditional tissue engineering methods just couldn’t achieve, and it’s why I get so excited about the possibilities.
From Concept to Creation: Bioprinting Techniques Taking Center Stage
When you look at the actual printing techniques, there’s quite a bit of variety, each with its own strengths. Extrusion-based bioprinting, for example, is incredibly popular because it can handle high-viscosity bio-inks and create complex tissue structures with high cell densities. It’s like squeezing toothpaste from a tube, but with incredible control and precision. Then you have inkjet-based printing, which offers super high precision and cost-efficiency for smaller tissue models, great for drug testing. Laser-assisted techniques are also gaining traction, particularly for their ability to ensure superior cell viability. What I find really fascinating is how these techniques are constantly being refined, with continuous innovations in areas like micro-extrusion and stereolithography, pushing the boundaries of what’s possible, especially in creating vascularized tissues and organ scaffolds. This technological diversity means that researchers have a robust toolkit to tackle different biological challenges, which I personally believe is vital for the rapid progress we’re seeing.
Healing and Repair: Bioprinting’s Impact on Regenerative Medicine
One of the most inspiring applications of bioprinting, for me, is its incredible potential in regenerative medicine. Think about all the people suffering from severe burns, chronic wounds, or damaged bones and cartilage. Bioprinting is literally opening up new avenues for healing and repair that were once unimaginable. It’s not just about patching things up; it’s about regenerating lost or damaged tissues with patient-specific solutions. I’ve read about breakthroughs where scientists are successfully regenerating hair follicles and skin appendages, which is huge for burn victims or people with hair loss. And it’s not just superficial tissues; engineers are even creating artificial bone materials and using bioprinting to advance bone grafting techniques. This is where the personalized aspect really shines, as bioprinting offers the opportunity to generate tissue made from a patient’s own cells, significantly reducing the risk of immune rejection, which is a massive hurdle in traditional transplants.
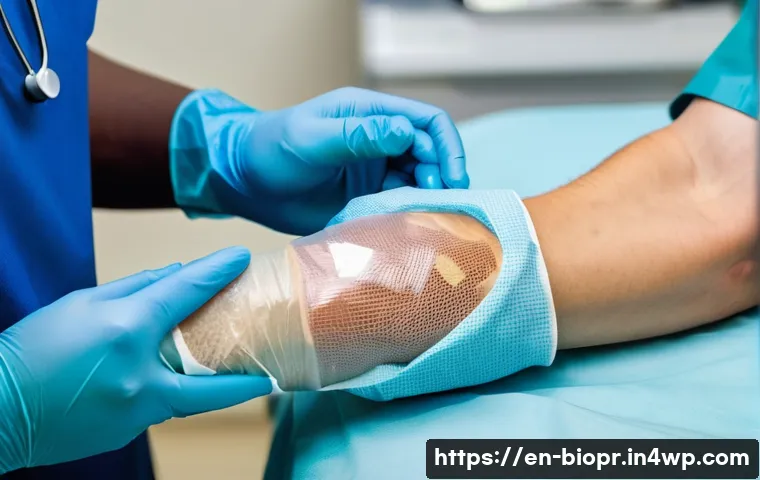
Skin Deep and Beyond: Revolutionizing Wound Care
When it comes to wound healing, bioprinting is already making waves. Imagine being able to print skin grafts directly onto a wound, tailored precisely to the patient’s needs. This is becoming a reality, with researchers developing multilayered skin consisting of both the dermis and epidermis. I’ve seen some incredible updates, like Inventia Life Science’s world-first in-situ skin bioprinting clinical trial, where they’re directly delivering patient-derived cells into gum and burn wounds. This kind of localized, on-demand treatment can proactively prevent infections and promote faster, more effective healing than ever before. It’s a complete game-changer for people suffering from extensive skin defects, offering hope for rapid, personalized surgical solutions that truly integrate with their body.
Rebuilding the Blueprint: Bones, Cartilage, and More
Beyond skin, the applications extend to our musculoskeletal system. The ability to bioprint bone grafts and cartilage structures is nothing short of amazing. Researchers are working on creating durable bone materials for replacement, offering solutions for complex fractures or conditions that require reconstructive surgery. I was particularly impressed by Penn State’s HITS-Bio platform, which can print cell spheroids at incredibly high speeds, leading to functional cartilage and bone repair in rats. This kind of innovation means we’re moving towards a future where damaged joints or bones could be repaired or even replaced with biological structures that integrate seamlessly, restoring full function. It’s a testament to how far we’ve come, moving from just envisioning these things to actively making them happen in the lab.
Accelerating Discovery: Bioprinting’s Role in Drug Development
One area where bioprinting is truly shining, and which has personally thrilled me, is in drug discovery and development. Historically, testing new drugs has been a slow, expensive process, often relying on animal models that don’t always accurately reflect human biology. But now, with bioprinting, we can create incredibly realistic 3D human tissue models and ‘organs-on-chips’ in a lab setting. These models are not just static structures; they can mimic the physiological functions and complex cellular interactions of actual human organs, offering a much more accurate and ethical platform for testing drug efficacy and toxicity. This means we can screen potential treatments much faster and bring them to clinical trials more quickly, potentially saving countless lives and accelerating medical breakthroughs. It’s a fundamental shift in how pharmaceuticals are developed.
Better Models, Better Drugs: Refining Pre-Clinical Testing
The beauty of bioprinted disease models lies in their ability to precisely replicate the cellular complexity and microenvironment of human tissues affected by various diseases. For example, scientists have used 3D bioprinting and patient stem cells to produce functional eye tissue to study diseases that cause blindness. This allows researchers to observe disease progression and test new therapies in a highly controlled, yet biologically relevant, environment. I truly believe that by moving away from archaic animal trials and towards these sophisticated human models, we’re not only making the process more humane but also significantly improving the predictive power of drug testing. It’s about getting better results, faster, and with more ethical considerations.
Personalized Pharmacology: Tailoring Treatments for Individuals
Beyond general drug screening, bioprinting is paving the way for truly personalized pharmacology. Imagine being able to test a range of drugs on a bioprinted tissue model derived directly from a patient’s own cells. This would allow doctors to see exactly how a particular individual’s body reacts to different medications, eliminating guesswork and dramatically improving treatment outcomes. Bioprinted drug-eluting patches, for instance, offer automated dosing and localized delivery, minimizing side effects often associated with systemic drug use. This level of customization can help increase dosing compliance, which is a common challenge with self-administered treatments. It feels like we’re on the cusp of a medical revolution where treatments are not just effective, but perfectly tailored for each and every one of us.
Navigating the Unknown: Overcoming Bioprinting’s Hurdles
As revolutionary as bioprinting is, it’s not without its challenges. Like any groundbreaking technology, there are significant hurdles we need to clear before it becomes a widespread clinical reality. From what I’ve seen, researchers are incredibly dedicated to pushing these boundaries, but it’s important to acknowledge the road ahead. One of the biggest obstacles is the sheer complexity of recreating the intricate architecture of human organs, which are composed of multiple cell types arranged in precise three-dimensional structures. Simply put, our bodies are masterpieces of natural engineering, and replicating that perfectly in a lab is no easy feat. Also, ensuring the long-term survival and functionality of cells after printing remains a significant hurdle; cells need the right microenvironment, biochemical signals, and physical cues to thrive, which can be tough to mimic.
The Vascularization Conundrum: Keeping Tissues Alive
Perhaps the most pressing technical challenge, especially for larger tissue constructs and organs, is vascularization – creating an intricate network of blood vessels to supply nutrients and oxygen and remove waste. Without a functional blood supply, larger bioprinted tissues just can’t survive for long. Traditional tissue engineering methods have fallen short in reproducing this complex 3D vascular network. While there have been remarkable advancements, such as 3D Systems’ print-to-perfusion system and Harvard’s Wyss Institute successfully printing vascularized human tissues on a chip, we’re still working on scaling this up for whole organs. It’s a deeply complex, multidisciplinary problem that requires a combination of emerging technologies to truly overcome. It’s like trying to build a city without any roads or plumbing – the infrastructure is just as vital as the buildings themselves.
Standardization, Regulation, and Scaling Up
Beyond the biological and engineering challenges, we also face practical and logistical hurdles. The development of suitable bio-inks that can consistently support cell growth and differentiation while maintaining structural integrity is an ongoing area of focus. We also need standardized bio-ink libraries coupled with quality control systems to ensure reproducibility, safety, and efficacy for specific applications. Then there’s the regulatory landscape, which is still evolving. Establishing a clear and defined regulatory pathway for bioprinted constructs is crucial for clinical translation, and addressing ethical implications like genetic privacy is also incredibly important. Scaling up production to meet widespread medical demand is another huge task. I mean, we’re talking about going from creating small tissue patches to potentially producing organs on demand, which is an enormous leap.
The Wonders of Bio-Inks: More Than Just Ink
If you’ve ever stopped to think about what actually goes into these incredible bioprinted structures, you’d realize the magic largely resides in what we call “bio-inks.” These aren’t just any old printer inks; they’re incredibly sophisticated materials, often hydrogel-based, that encapsulate living cells and provide the perfect microenvironment for them to grow, differentiate, and organize. They’re essentially the scaffolding and the nutrient-rich goo that keeps everything alive and happy during and after the printing process. When I first learned about how specifically engineered these bio-inks have to be, I was honestly blown away. It’s a delicate balance of mechanical properties, biocompatibility, and the ability to degrade naturally over time, allowing the patient’s own cells to take over.
Natural vs. Synthetic: The Perfect Blend
The innovation in bio-ink development is truly mind-boggling. Researchers are constantly experimenting with a wide array of materials, both natural and synthetic, to create the ideal compositions. Natural polymers like alginate, collagen, silk, and gelatin are popular choices because they mimic the body’s natural extracellular matrix and show promising results for cellular activity and tissue functionality. But then you also have synthetic polymer-based hydrogels, which offer the flexibility to design specific molecular structures and chemical modifications, giving engineers more control over properties like mechanical strength and degradation rates. What’s really exciting is the development of hybrid bio-inks, combining the best of both worlds – the biocompatibility of natural materials with the tunable properties of synthetic ones – for creating multi-functional tissues and complex scaffolds. This quest for the “perfect bio-ink” is a critical frontier in bioprinting, and it’s something I always keep an eye on because new developments here can unlock so many possibilities.
Tailoring Bio-Inks for Specific Tissues
The sheer variety of tissues we want to print means there’s no one-size-fits-all bio-ink. Think about it: skin needs different properties than bone or heart tissue. So, scientists are working on tailoring bio-inks for specific applications. For example, for bone repair, they might use composites with boron-based glass and polycaprolactone to create durable scaffolds that encourage cell adhesion and promote tissue repair. For heart tissue, the bio-ink stiffness and initial print geometry can even be modified to encourage “shape-morphing,” allowing tissues to change shape due to cell-generated forces, mimicking natural embryonic development and improving structural and functional maturity. It’s all about creating an environment that encourages the cells to do what they do best: build and repair, but with a little intelligent guidance from the bio-ink. This targeted approach is what makes personalized medicine so powerful, ensuring that each printed tissue gets exactly what it needs to thrive.
AI and Automation: Supercharging Bioprinting’s Potential
Here’s where it gets even more futuristic, and where I personally feel the real acceleration is happening: the integration of Artificial Intelligence (AI) and machine learning into the bioprinting workflow. If you thought bioprinting alone was incredible, adding AI to the mix is like giving it a superpower. AI is revolutionizing nearly every stage of bioprinting, from designing complex tissue structures to optimizing printing parameters and even automating the entire process. It’s all about handling vast datasets, performing complex computations, and learning from historical data to make the whole process smarter, faster, and remarkably more efficient. This synergy is precisely why we’re seeing such rapid advancements in the field, and it’s a huge factor in speeding up the development cycle, which is essential for getting these life-changing technologies to patients.

Intelligent Design: Crafting Tissues with Unprecedented Precision
Imagine designing a complex organ with hundreds of different cell types and intricate vascular networks. Manually, that’s an almost impossible task. But AI algorithms can analyze medical imaging data and patient-specific information to design custom tissue and organ structures with unprecedented precision. They can help determine the ideal cell types, concentrations, and spatial arrangements needed for specific tissue types, enhancing the viability and functionality of the printed constructs. I’ve been reading about how AI-driven process optimization can reduce bioprinting development time by a significant 25-30% and even cut defect rates by up to 30% in scaffold manufacturing systems. This data-driven approach isn’t just about speed; it’s about a level of accuracy and customization that was previously unattainable, bringing us closer to truly functional, implantable tissues. It’s almost like having an incredibly intelligent co-pilot for the entire bioprinting journey.
Real-Time Optimization and Automated Workflows
The impact of AI isn’t just in the design phase; it extends to real-time monitoring and optimization during the printing process itself. AI algorithms can dynamically adjust printing parameters, like speed and pressure, to improve fidelity and reduce waste. This means the bioprinter can essentially “learn” and adapt on the fly, ensuring that each layer is laid down perfectly and the cells remain viable. Machine learning, in particular, has a significant impact by analyzing large datasets to improve techniques and predict the behavior of bioprinted constructs. This automation streamlines workflows, reduces reliance on resource-intensive trial-and-error experimentation, and ultimately makes the entire bioprinting process more sustainable and scalable. It’s truly incredible to see how these advanced technologies are converging to accelerate innovation and bring us closer to a future where bioprinting is a routine part of medicine.
From Lab Bench to Bedside: The Road to Clinical Translation
While the scientific advancements in bioprinting are absolutely incredible, the real test, for me, is when these innovations can actually reach patients. The journey from a groundbreaking discovery in a lab to a widely available clinical treatment is long and filled with its own unique set of challenges. It’s not enough to print a functional tissue in a petri dish; we need to ensure it can be safely and effectively implanted into a human body, function as intended for the long term, and be produced on a scale that can meet massive medical demand. This is where clinical translation comes in, and it’s a phase that requires intense collaboration between scientists, engineers, clinicians, and regulatory bodies.
Pilot Programs and Early Success Stories
Despite the hurdles, we’re already seeing exciting progress in clinical translation. I get so hopeful when I hear about successful pilot programs and early clinical trials. For instance, that in-situ skin bioprinting clinical trial I mentioned earlier? It’s actively treating patients right now. And there are already FDA-approved bioprinted medical devices entering commercial markets, like the COAPTIUM CONNECT for nerve repair, which boasts 100% surgical success in early applications. These aren’t just isolated incidents; they’re strong signals of commercial confidence and represent crucial steps towards broader clinical adoption. Seeing these early successes really drives home the idea that this isn’t just abstract science; it’s actively improving lives today.
Overcoming Regulatory and Scalability Challenges
One of the less glamorous but incredibly vital aspects of clinical translation is navigating the regulatory landscape. Because bioprinting is such a new and complex field, establishing clear guidelines for safety, efficacy, and quality control is an ongoing process. We need Good Manufacturing Practice (GMP) grade containment systems and bio-ink materials to ensure sterility and consistency, which are currently limited. Then there’s the challenge of scalability. How do we go from printing a few tissues for research to mass-producing organs for transplantation? This involves significant investment in infrastructure, automation, and advanced manufacturing techniques. It’s a massive undertaking, but with increasing investment flows and collaborative consortia forming across academia, industry, and regulatory bodies, I’m optimistic that we’ll continue to accelerate these translational pipelines and make bioprinting a routine part of modern medicine.
The Future is Now: Peeking into Bioprinting’s Horizon
If you’ve followed along this far, you can probably tell I’m incredibly excited about where bioprinting is headed. While we’ve already seen incredible breakthroughs, it honestly feels like we’re just scratching the surface of what’s possible. The pace of innovation is accelerating, and the predictions for the coming years are nothing short of astounding. I truly believe bioprinting is poised to initiate a paradigm shift in how we approach healthcare, from treating chronic diseases to extending human lifespan and improving our overall quality of life. It’s a field that constantly reminds me of the boundless potential of human ingenuity.
Full Organ Bioprinting: The Ultimate Dream
The ultimate goal, the holy grail of bioprinting, is undoubtedly the ability to print entire, fully functional human organs for transplantation. Imagine a world where the agonizing waitlist for organ donors becomes a thing of the past, replaced by on-demand, patient-specific organs. Researchers are actively working towards this, with some even aiming to bioprint an entire living, working human heart. While we’re still a ways off from large-scale, implantable organs, the progress in creating complex liver structures, heart valves, and even living heart tissues is incredibly promising. The key will be perfecting the vascular networks within these larger structures, allowing them to remain viable and integrate seamlessly with the body. I honestly feel that with continued dedication and innovation, this dream will eventually become a reality, profoundly transforming transplant medicine.
Beyond Replacement: Enhanced Human Capabilities
But what if bioprinting goes beyond just repairing and replacing? What if it could actually enhance human capabilities? This is where my imagination really starts to run wild. We’re already seeing advances that could lead to “smart” drug delivery devices bioprinted to interact safely with a patient’s biological system, or innovative wound-healing protocols that proactively prevent infections. Could we one day bioprint tissues with enhanced properties, making them more resilient to disease or injury? The thought is both thrilling and a little bit daunting, pushing the boundaries of what it means to be human. It’s a future where regenerative medicine isn’t just about restoring health, but about unlocking new potentials for human biology. The continuous development of multi-material and multi-cell printing, alongside the integration of advanced technologies like AI, makes me believe that this visionary future might arrive sooner than we think.
| Bioprinting Application | Current Status / Key Breakthroughs | Impact on Patients |
|---|---|---|
| Personalized Organ Models | Functional liver, heart, and eye tissues for disease modeling. | More accurate drug testing, reduced need for animal trials, personalized treatment strategies. |
| Skin Regeneration | In-situ bioprinting clinical trials for burn and wound healing. Multilayered skin grafts. | Faster, more effective wound healing, reduced scarring, personalized skin replacement. |
| Bone & Cartilage Repair | Artificial bone materials, functional cartilage repair in animal models. | Improved recovery from injuries, potential for joint reconstruction, patient-specific implants. |
| Drug Delivery Devices | Bioprinted drug-eluting patches, smart drug delivery systems. | Automated, localized drug delivery, minimized side effects, improved patient compliance. |
| Full Organ Transplantation | Complex liver structures, heart valves, vascularized tissue models. | Future elimination of donor shortages, reduced immune rejection, on-demand organs. |
Wrapping Things Up
And there you have it, folks! My deep dive into the incredible world of bioprinting. Honestly, every time I research this topic, I’m left buzzing with excitement and a genuine sense of wonder at human ingenuity. What started as a science fiction dream is rapidly evolving into a tangible reality, and it’s truly going to redefine what’s possible in medicine. We’re talking about a future where healing is not just about recovery, but about genuine regeneration and personalized care. It’s a journey filled with awe-inspiring breakthroughs and persistent challenges, but the trajectory is undeniably upward. Keep an eye on this space, because I have a strong feeling we’re only just beginning to see the profound impact bioprinting will have on all our lives.
Useful Information to Know
1. Staying Ahead of the Curve in Bioprinting Innovation: This field moves at a blistering pace, and it can feel a bit like trying to catch lightning in a bottle! If you’re as fascinated as I am, you’ll want to know where to find the freshest insights. I’ve personally found that following reputable scientific journals like “Nature Biomedical Engineering” or “Science Translational Medicine” is crucial, but for more digestible, real-time updates, key tech news sites and specialized biotech blogs often break down complex research into exciting headlines. Also, keep an eye out for virtual conferences or webinars hosted by organizations like the International Society for Bioprinting (ISB). They often feature pioneers sharing their latest discoveries, offering a fantastic glimpse into what’s just over the horizon. It’s truly inspiring to see the global community pushing these boundaries together.
2. Exploring Career Paths in Bioprinting: For any of you out there considering a future in science or engineering, bioprinting represents an incredibly vibrant and multidisciplinary career landscape. It’s not just for doctors or traditional biologists; this field thrives on collaboration. You’ll find opportunities for biomedical engineers designing the printers and bio-inks, material scientists developing novel biocompatible compounds, cell biologists culturing and preparing living cells, and even computer scientists crafting the AI algorithms that optimize printing processes. Clinical researchers are vital for testing new bioprinted tissues, and let’s not forget the ethical and regulatory experts who ensure these innovations are brought to patients safely and responsibly. My advice? If you’re passionate about making a tangible difference in human health, explore the intersection of these disciplines – that’s where the magic truly happens!
3. Navigating the Ethical Labyrinth of Bioprinting: While the promises of bioprinting are incredibly exciting, it’s also important to acknowledge the profound ethical considerations that come with engineering life itself. Questions arise about the moral status of bioprinted tissues, especially as they become more complex and organ-like. There’s also the crucial issue of equitable access: will these groundbreaking, potentially life-saving technologies be available to everyone, or only a privileged few? And as we delve into patient-specific organs, discussions about genetic privacy and the potential for enhancement over mere repair become incredibly relevant. These aren’t easy questions, and I believe it’s vital for scientists, ethicists, policymakers, and the public to engage in thoughtful dialogue now, ensuring that our technological advancements are guided by strong moral principles.
4. Demystifying Bio-Inks: The Unsung Heroes of Bioprinting: When we talk about bioprinting, the “ink” is fundamentally different from what you’d find in a conventional 3D printer or even your home inkjet. Forget plastic or metal; bio-inks are typically hydrogel-based materials, often infused with living cells, growth factors, and other biomolecules. They are ingeniously designed to mimic the natural extracellular matrix of human tissues, providing a supportive, nutrient-rich environment for cells to thrive during and after printing. The challenge lies in creating an ink that has the right mechanical properties for printing, is biocompatible (doesn’t harm the cells), and can eventually degrade or integrate seamlessly with the host tissue. It’s a delicate dance of chemistry and biology, and the innovation in this area is what truly unlocks the potential for functional, living tissues and organs.
5. Bioprinting vs. Traditional 3D Printing: A Key Distinction: It’s easy to conflate bioprinting with the general concept of 3D printing, but there’s a critical difference that’s worth highlighting. Traditional 3D printing creates inert, non-living objects from materials like plastic, metal, or resin, purely for structural or aesthetic purposes. Bioprinting, on the other hand, is about fabricating living, functional biological structures using living cells and biomaterials. The ultimate goal isn’t just a physical shape, but a structure that can mimic physiological functions, interact with a biological system, and ideally, integrate into a living body. This focus on viability, function, and biological interaction means the precision, materials, and processes involved are infinitely more complex and exciting than simply printing a plastic toy.
Key Takeaways
So, what’s the big picture here? Bioprinting is absolutely revolutionary, poised to transform healthcare as we know it. We’re talking about a future where personalized medicine truly comes alive, offering patient-specific solutions for everything from severe burns to damaged organs, significantly reducing the risks of immune rejection that plague traditional transplants. This isn’t just about repairing; it’s about regenerating and, potentially, even enhancing. The integration of advanced technologies like AI and machine learning is supercharging this field, making design more precise and processes more efficient than ever before. While significant challenges like creating complex vascular networks and navigating regulatory pathways remain, the incredible progress and accelerating pace of innovation, coupled with increasing investments, suggest that bioprinting is not just a distant dream, but a rapidly approaching reality that will redefine our understanding of health and the human body.
Frequently Asked Questions (FAQ) 📖
Q: What exactly can bioprinting do right now? I mean, beyond the sci-fi movie scenes, what’s genuinely happening in labs and clinics?
A: Oh, this is such a fantastic question, and one I get all the time! From my personal deep dives and conversations with folks in the field, what’s happening right now is genuinely transformative.
We’re not just talking about theory anymore. Bioprinting is already revolutionizing drug discovery and testing. Instead of relying on traditional animal trials that don’t always translate perfectly to humans, researchers are literally printing miniature, functional human organ models – think tiny livers or kidneys.
This means they can test new medications with far greater accuracy and speed, which is a massive leap forward. Beyond that, the ability to create custom-tailored skin grafts for burn victims is becoming a reality, offering such a personalized approach to healing.
And it’s not just basic structures; we’re seeing incredible progress in engineering complex tissues that truly mimic our body’s own intricate designs.
It’s truly amazing to witness how quickly these advancements are moving from concept to tangible, life-changing applications. It really feels like we’re on the cusp of something monumental!
Q: That sounds incredible! But how does bioprinting actually work? What’s the magic behind turning cells into complex tissues?
A: I love this question because it gets to the heart of the ingenious process! When I first learned about it, I was totally blown away. Imagine a highly specialized 3D printer, but instead of plastic or metal, it’s using “bio-inks.” These aren’t your typical inks; they’re remarkable formulations packed with living cells, growth factors, and biomaterials that act as a scaffold.
The printer then meticulously deposits these bio-inks, layer by tiny layer, to build up a 3D structure. Think of it like building with microscopic, living LEGOs!
What’s truly pushing the boundaries now is the seamless integration of AI. Artificial intelligence helps refine the precision of cell placement, optimize the structure’s integrity, and even predict how the cells will behave once printed, making the entire process smarter, faster, and incredibly more efficient.
It’s a delicate dance of engineering, biology, and cutting-edge computing, and seeing it in action is absolutely mesmerizing.
Q: With all this amazing progress, what are the biggest hurdles bioprinting still needs to overcome before it becomes a common medical treatment?
A: Ah, yes, the inevitable challenges! While the momentum is absolutely electrifying, it’s important to be real about the roadblocks. From what I’ve observed and heard from experts, one of the biggest challenges right now is scaling up production.
Creating a small patch of tissue in a lab is one thing, but producing a full, functional organ on demand for widespread clinical use? That’s a whole different ball game.
Another huge hurdle is achieving proper vascularization – essentially, creating a functional blood supply within these printed tissues. Without it, the cells can’t get the nutrients they need to survive and thrive.
Then there’s the monumental task of regulatory approval; ensuring these printed tissues are safe and effective for human implantation is a long and rigorous process.
And, let’s not forget the immune response. We need to ensure the body doesn’t reject these incredible, bio-engineered creations. It’s a complex puzzle, but honestly, the sheer brainpower and dedication pouring into solving these issues give me immense hope.
We’re definitely not there yet for every application, but every day brings us closer!