Have you ever stopped to truly consider the sheer potential of regenerating human tissue? It feels like something straight out of a sci-fi movie, yet we’re witnessing its rapid progression right before our eyes.
The convergence of bioprinting and stem cell research isn’t just a fascinating academic topic; it’s genuinely reshaping the future of medicine as we know it.
I remember the first time I grasped the concept – using 3D printing to create functional biological structures with the very cells that can become *anything*.
That’s where the magic truly begins, and it’s powered by the incredible versatility of stem cells. This isn’t just about growing a new ear or repairing damaged cartilage, although those breakthroughs are incredible.
We’re talking about tackling massive challenges like organ scarcity, developing personalized drug testing platforms that could revolutionize pharmaceutical trials, and even creating complex disease models outside the human body for unprecedented research insights.
The momentum behind this field is palpable, driven by cutting-edge advancements in AI and material science, making it truly feel like we’re on the cusp of eliminating transplant waiting lists entirely.
It’s an electrifying thought, isn’t it? Let’s peel back the layers and explore this transformative field in detail.
Building the Future, Cell by Cell: The Bioprinting Revolution
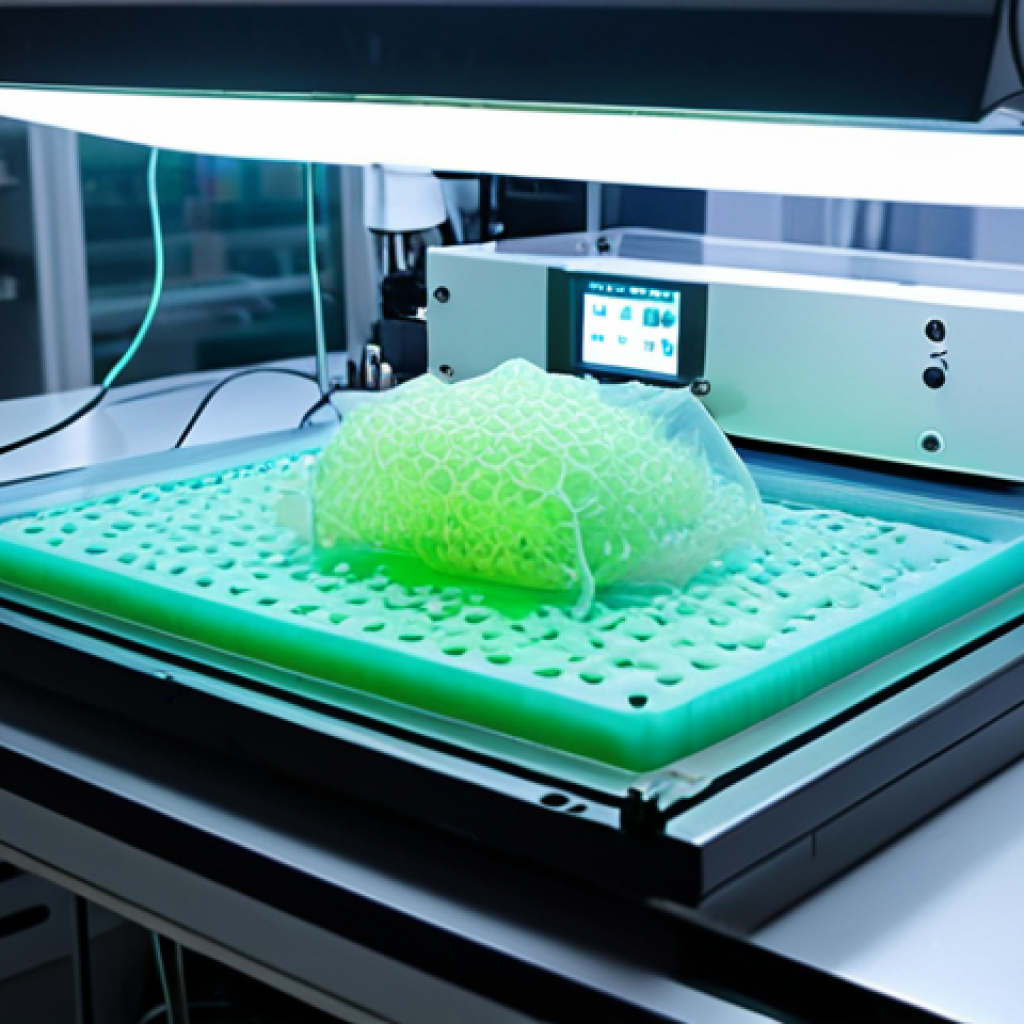
Stepping into the world of bioprinting feels a bit like peeking into a future that’s already here, unfolding before our very eyes. When I first encountered the concept, I honestly thought it sounded like something plucked straight from a high-tech movie script – the idea of printing living tissues, even organs, layer by intricate layer. But the reality is far more astounding and profoundly impactful than any cinematic fantasy. Bioprinting is, at its core, an additive manufacturing process, but instead of plastic or metal, it uses “bio-inks” comprised of living cells, growth factors, and biomaterials. Imagine precisely depositing these biological building blocks with the kind of accuracy you’d expect from a high-end 3D printer, constructing complex structures that mimic the intricate architecture of human tissues. It’s a painstaking process, requiring immense precision and a deep understanding of cellular biology, but the potential payoff is truly monumental. From creating small patches of skin to engineering complex vascularized tissues, the field is rapidly expanding its capabilities, pushing the boundaries of what we once thought was medically impossible. The challenges are significant, of course, from ensuring cell viability during printing to achieving the structural integrity and functionality required for medical applications, but the dedication of researchers worldwide is simply inspiring.
The Art and Science of Bio-Ink Formulation
One of the most critical components in the bioprinting puzzle is the “bio-ink” itself. This isn’t just a generic goo; it’s a carefully engineered mixture that needs to support cell survival, proliferation, and differentiation, all while being printable. Think about it: you need a material that’s viscous enough to hold its shape after extrusion but fluid enough to flow through tiny nozzles. On top of that, it has to be biocompatible, meaning it won’t harm the cells, and ideally, biodegradable, so it can eventually degrade and be replaced by the body’s own newly formed tissue. Researchers are constantly experimenting with various hydrogels, natural polymers like alginate and collagen, and synthetic polymers, often incorporating growth factors and signaling molecules to encourage specific cellular behaviors. It’s a delicate balance, trying to find the perfect blend that offers both mechanical support and biological cues. I’ve seen presentations where scientists talk about engineering these bio-inks at a molecular level, tailoring them for specific tissue types – a true testament to the interdisciplinary nature of this field, blending material science, biology, and engineering.
Precision Layering: How Bioprinters Work Their Magic
The actual bioprinting process relies on several sophisticated techniques, each with its own advantages and challenges. Extrusion-based bioprinting is perhaps the most common, pushing bio-ink through a nozzle onto a platform, similar to traditional FDM 3D printing. Then there’s inkjet bioprinting, which uses tiny droplets of bio-ink, allowing for incredibly high resolution and precision, much like an office inkjet printer. Laser-assisted bioprinting offers even finer control, using a focused laser to deposit cells with unparalleled accuracy. Each method brings unique capabilities to the table, and often, hybrid approaches are used to combine their strengths. The goal is always the same: to place cells in exactly the right location, in the correct density, within a supportive scaffold, to encourage them to grow and organize into functional tissue. It’s mind-boggling to think about the level of control required – not just over the printer head, but over the microscopic environment of living cells as they are being assembled. When you see a time-lapse of a simple tissue being printed, it truly hits home just how revolutionary this technology is.
Stem Cells: Unlocking the Body’s Innate Regenerative Power
If bioprinting is the advanced construction tool, then stem cells are undoubtedly the master builders – the true magic behind regenerative medicine. These aren’t just any cells; they possess an extraordinary ability to self-renew and to differentiate into various specialized cell types, essentially acting as the body’s repair kit. When I first learned about embryonic stem cells, their pluripotency – the capacity to become *any* cell in the body – felt like a revelation, both scientifically profound and ethically complex. However, the field has evolved incredibly rapidly. The discovery of induced pluripotent stem cells (iPSCs) was a monumental breakthrough, allowing scientists to reprogram adult somatic cells (like skin cells!) back into an embryonic-like state. This completely sidestepped many of the ethical concerns associated with embryonic stem cells and opened up a vast new avenue for personalized medicine. Imagine being able to take a patient’s own skin cells, reprogram them, and then use those reprogrammed cells to grow new tissue or even organs that are genetically identical to the patient – eliminating the risk of immune rejection, which is a massive hurdle in traditional organ transplantation. It’s a game-changer, plain and simple.
Navigating the Ethical Landscape and Advancements in iPSCs
The journey with stem cells has always been intertwined with significant ethical considerations, particularly concerning embryonic stem cell research. For many years, this was a highly contentious area, prompting vital discussions about the moral status of embryos. However, the advent of induced pluripotent stem cells truly revolutionized the conversation. The ability to derive these “pluripotent” cells from adult tissue, rather than embryos, has opened doors that were previously closed, allowing for a broader consensus and accelerated research. I’ve personally followed the incredible progress in iPSC technology, from the initial discovery by Shinya Yamanaka and Kazutoshi Takahashi to the increasingly efficient and safer methods of reprogramming cells. This advancement means we can now generate patient-specific cell lines for disease modeling, drug screening, and even therapeutic applications without the ethical dilemmas that once slowed down the field. It’s a testament to how scientific innovation can sometimes provide elegant solutions to complex societal challenges.
The Versatility of Stem Cells in Bioprinting
The true power couple in regenerative medicine is the combination of bioprinting and stem cells. Stem cells provide the raw material – the living, adaptable cells – while bioprinting provides the architectural precision to arrange them into functional structures. Think about what this means:
- Organoids and Tissue Models: We can print mini-organs or complex tissue constructs that accurately mimic human physiology for drug testing and disease research, reducing the need for animal testing.
- Personalized Implants: Imagine a patient needing a cartilage repair. We could take their own iPSCs, differentiate them into chondrocytes (cartilage cells), and then bioprint a custom-fit cartilage patch.
- Bridging Gaps in Damage: For large tissue defects, whether from trauma or disease, bioprinted scaffolds seeded with stem cells can act as a bridge, guiding new tissue growth and regeneration.
The synergy between these two technologies is what makes the field so exhilarating. It’s not just about growing cells in a dish; it’s about giving them a proper, architecturally sound home where they can perform their biological functions as they would in the human body. This capability fundamentally changes our approach to treating a vast array of diseases and injuries.
Beyond the Lab Bench: Real-World Impacts on Healthcare
While much of the groundbreaking work in bioprinting and stem cell research happens in highly specialized laboratories, the ultimate goal is always translation to real-world clinical applications. And let me tell you, the potential impact on healthcare is nothing short of revolutionary. We’re not just talking about incremental improvements; we’re talking about fundamental shifts in how we treat chronic diseases, heal injuries, and even perform drug development. The most immediate and compelling application, for me, is the promise of personalized medicine. Imagine a future where treatments are tailored not just to your genetic makeup, but to your specific, individual cellular biology. This isn’t science fiction anymore; it’s becoming a tangible reality driven by these technologies. From creating custom tissues for burn victims to developing disease models that respond exactly like a specific patient’s disease would, the implications are vast and deeply personal. It’s an exciting time to be involved in or simply observing the progression of this field.
Revolutionizing Organ Transplantation and Scarcity
One of the most pressing challenges in modern medicine is the severe shortage of donor organs. Thousands of people worldwide are on transplant waiting lists, and tragically, many die before a suitable organ becomes available. This is where bioprinting, especially with stem cells, offers a beacon of hope. The long-term vision is to bioprint functional organs – kidneys, livers, hearts – that are genetically matched to the patient, thereby eliminating the need for immunosuppressive drugs and significantly reducing rejection rates. While a fully functional, vascularized organ is still some way off, significant strides are being made with simpler tissues and organ components. Even printing functional patches for a damaged heart, or liver tissue for detoxification, could dramatically improve patient outcomes and alleviate the strain on donor networks. This is a future I genuinely believe we will see, and it will save countless lives.
Accelerating Drug Discovery and Personalized Treatment
The pharmaceutical industry currently relies heavily on animal models and 2D cell cultures for drug testing, which often fail to accurately predict how a drug will behave in the complex human body. Bioprinted 3D tissue models, derived from human cells – perhaps even patient-specific iPSCs – are changing this paradigm. These “organs-on-a-chip” or “disease-in-a-dish” models provide a far more accurate representation of human physiology and pathology, allowing researchers to:
- Screen Drugs More Effectively: Test new compounds on realistic human tissues, identifying effective treatments and potential toxicities much earlier in the development process.
- Personalize Therapies: For patients with complex conditions like cancer, bioprinted models of their specific tumors can be used to test various drugs and identify the most effective treatment plan for them, minimizing trial-and-error.
- Reduce Animal Testing: A significant ethical and practical benefit, moving towards more human-relevant testing platforms.
I find this particularly thrilling because it promises to not only make drug development faster and cheaper but also much safer and more tailored for individual patients. The era of one-size-fits-all medicine is slowly, but surely, giving way to an era of hyper-personalized care.
Addressing the Ethical Labyrinth and Regulatory Hurdles
As exciting as the future of bioprinting and stem cells is, it’s crucial to acknowledge the complex ethical and regulatory landscape that accompanies such revolutionary technologies. When you’re talking about creating human tissues outside the body, or even modifying cells, it raises profound questions that society needs to grapple with. It’s not just about what we *can* do, but what we *should* do, and how we ensure these advancements are used responsibly and equitably. From the ethical sourcing of cells to the long-term implications of engineered tissues, there are no easy answers, and open, honest dialogue is paramount. Regulatory bodies, often slow to adapt to rapidly evolving science, are also playing catch-up, trying to establish frameworks that ensure safety and efficacy without stifling innovation. It’s a delicate dance, but one that is absolutely essential for the healthy progression of this field.
Ensuring Responsible Innovation and Patient Safety
The primary concern for any medical breakthrough is patient safety. With bioprinted tissues and stem cell therapies, this means rigorous testing for functionality, longevity, and potential unintended side effects. What if a bioprinted organ doesn’t integrate properly? What are the risks of long-term immune responses, even with patient-specific cells? These are questions that demand exhaustive preclinical and clinical trials. Regulatory bodies like the FDA in the US or the EMA in Europe are working to establish clear guidelines for these novel therapies, but the unique nature of living constructs means traditional drug or device approval pathways don’t always fit perfectly. I’ve heard researchers express frustration with the pace of regulation, but I also understand the immense responsibility involved. Balancing the urgency of patient need with the necessity of thorough safety validation is a continuous challenge that requires collaboration between scientists, ethicists, and policymakers.
Equity and Accessibility: Avoiding a Two-Tiered Future
Beyond safety, perhaps the biggest ethical challenge is ensuring that these transformative therapies are accessible to everyone, not just a privileged few. Regenerative medicine is incredibly expensive to develop and, initially, will likely be very costly to implement. The last thing anyone wants to see is a future where only the wealthy can afford life-saving bioprinted organs or personalized stem cell treatments. This raises critical questions about healthcare policies, insurance coverage, and global collaboration to share knowledge and resources. It’s something I think about often: how do we ensure that breakthroughs for humanity truly benefit all of humanity? The industry needs to be proactive in exploring cost-reduction strategies and advocating for equitable access from the very beginning, rather than waiting for disparities to become entrenched. The potential for a “medical haves and have-nots” scenario is a serious concern that warrants immediate attention.
The Economic and Societal Ripple Effect of Regenerative Medicine
When we talk about revolutionary medical advancements, it’s not just about the science; it’s about the profound impact they have on our economies and societies. Regenerative medicine, powered by bioprinting and stem cells, is poised to create a significant ripple effect that will touch everything from healthcare expenditures to employment markets and even our fundamental understanding of aging and disease. Think about the economic burden of chronic diseases and organ failure today. If we can bioprint functional replacements or regenerate damaged tissues, the long-term healthcare costs associated with managing these conditions could plummet. Fewer hospitalizations, less reliance on lifelong medications, and a healthier, more productive workforce – the economic benefits are staggering. And it’s not just about savings; it’s about new industries emerging, creating high-tech jobs in research, manufacturing, and clinical application. This isn’t just a medical revolution; it’s an economic paradigm shift in the making.
Transforming Healthcare Models and Reducing Long-Term Costs
The current healthcare system is often reactive, focused on managing symptoms and diseases once they’ve taken hold. Regenerative medicine offers the tantalizing prospect of proactive, curative approaches. Imagine if we could repair or replace a failing organ before it reaches end-stage disease, or prevent chronic conditions by regenerating damaged tissues early on. This shift from managing illness to restoring health could dramatically reduce the long-term financial strain on healthcare systems globally. While the initial investment in research and development is substantial, the downstream savings from fewer surgeries, reduced need for long-term care, and decreased reliance on immunosuppressants or palliative treatments could be immense. It’s a fundamental re-evaluation of how we allocate healthcare resources, moving towards a model focused on true restoration rather than perpetual management. I believe this will be a massive driver for widespread adoption once the technologies mature.
New Industries and Job Creation in a Biotech Boom
Every major technological leap creates new economic ecosystems, and regenerative medicine is no exception. This field isn’t just about laboratory research; it involves sophisticated engineering, advanced manufacturing, specialized clinical procedures, and complex supply chains. We’re already seeing the emergence of companies dedicated solely to bioprinting hardware, bio-ink development, stem cell banking, and regenerative therapy delivery. This boom is creating a surge in demand for highly skilled professionals across multiple disciplines:
| Sector | Key Roles | Potential Impact |
|---|---|---|
| Research & Development | Cell Biologists, Biomedical Engineers, Material Scientists | Driving innovation, discovering new therapies |
| Manufacturing & Production | Bioprinting Technicians, Quality Control Specialists | Scaling up production of tissues and organs |
| Clinical Applications | Regenerative Medicine Surgeons, Cell Therapists | Delivering treatments directly to patients |
| Regulatory & Policy | Bioethicists, Healthcare Lawyers, Regulatory Affairs Specialists | Ensuring safety, access, and ethical conduct |
This expansion will lead to significant job creation, from the scientists designing the next generation of bio-inks to the engineers building the advanced bioprinters, and the clinicians who will eventually deliver these life-changing therapies. It’s an exciting prospect for economic growth, fostering innovation hubs and attracting investment in biotech clusters around the world. The shift isn’t just medical; it’s a profound economic transformation.
Personalized Medicine’s New Frontier: Tailored Treatments for Every Body
For me, one of the most exciting promises of bioprinting and stem cell research lies in its capacity to usher in a new era of truly personalized medicine. Forget the one-size-fits-all approach that often characterizes current treatments. Imagine therapies designed not just for a disease, but specifically for *your* disease, *your* body, and *your* unique cellular makeup. This level of customization has been a long-held dream in medicine, and now, with the ability to engineer tissues and even organs from a patient’s own cells, it’s becoming a tangible reality. The potential to eliminate immune rejection, a major hurdle in current transplantation, by using autologous (patient-derived) cells is nothing short of revolutionary. This isn’t just about efficacy; it’s about making treatments safer, more targeted, and ultimately, far more effective for each individual. It’s a shift from treating a diagnosis to treating a person.
Eliminating Immune Rejection with Autologous Cells
The immune system is a powerful guardian, designed to protect us from foreign invaders. Unfortunately, in organ transplantation, it often sees the donated organ as a foreign threat, leading to rejection. This necessitates lifelong immunosuppressive drugs, which come with their own set of serious side effects, from increased risk of infection to kidney damage. The beauty of using induced pluripotent stem cells (iPSCs) derived from the patient themselves is that the resulting bioprinted tissues or organs are genetically identical to the recipient. This effectively renders the immune system inert, as it recognizes the new tissue as “self.” The implications are massive:
- No Immunosuppressants: Patients would be spared the lifelong burden and risks associated with these powerful drugs.
- Better Long-Term Outcomes: Organs and tissues would integrate more seamlessly and function optimally for longer periods.
- Reduced Complications: Fewer infections, kidney issues, and other common side effects seen in transplant patients.
This truly feels like the ultimate goal in tissue engineering – creating perfectly matched, fully functional biological replacements without the threat of rejection. It’s a vision that fuels immense hope for millions suffering from organ failure.
Creating Disease-Specific Models for Targeted Therapies
Another incredible aspect of personalized medicine enabled by these technologies is the ability to create highly specific disease models. Instead of relying on general cell lines or animal models, researchers can take cells from a patient with a particular disease (e.g., Alzheimer’s, Parkinson’s, specific cancers), reprogram them into iPSCs, and then differentiate them into the exact cell type affected by the disease. These patient-specific cells can then be used to:
- Understand Disease Mechanisms: Study how a disease progresses in a patient’s own cells, identifying unique pathological pathways.
- Screen Drugs for Individual Patients: Test various therapeutic compounds on these patient-specific models to see which one is most effective *for that individual*.
- Identify Biomarkers: Discover unique markers that can help diagnose the disease earlier or predict treatment response.
This allows for a level of diagnostic precision and therapeutic tailoring that was previously unimaginable. For instance, imagine being able to test dozens of chemotherapy drugs on a bioprinted model of a patient’s actual tumor, seeing which ones kill *their* cancer cells most effectively, before administering a single dose to the patient. That’s not just personalized medicine; that’s revolutionary, targeted healing.
From Bench to Bedside: Accelerating the Path to Clinical Reality
It’s one thing to make incredible discoveries in the lab, but it’s an entirely different, and often far more challenging, endeavor to translate those breakthroughs into treatments that benefit actual patients. The journey from “bench to bedside” in regenerative medicine is a complex one, fraught with hurdles related to scalability, standardization, safety, and regulatory approval. Yet, the momentum is palpable. We’re seeing more and more bioprinted constructs and stem cell therapies entering clinical trials, moving closer to becoming standard medical practice. This accelerated pace is driven by significant investment, global collaboration, and an undeniable societal need for more effective treatments for chronic and life-threatening conditions. While there are still mountains to climb, the progress in just the last decade has been nothing short of astonishing, giving us real hope that these therapies will soon be widely available.
Scaling Up Production for Widespread Availability
One of the biggest practical challenges is scaling up the production of bioprinted tissues and stem cell therapies to meet the demands of a global patient population. What works in a small lab setting needs to be reproducible, consistent, and cost-effective at a much larger scale. This involves developing automated bioprinting systems, optimizing cell culture processes to yield billions of cells, and establishing robust quality control measures to ensure every batch is safe and effective. It’s a transition from artisanal craftsmanship to industrial-level manufacturing, and it requires entirely new approaches to bioengineering and bioprocessing. I’ve heard many researchers emphasize that the scientific breakthrough is often just the first step; the engineering and manufacturing challenges that follow can be just as, if not more, demanding. But with dedicated efforts, we’re seeing impressive progress in moving towards scalable solutions.
Navigating Clinical Trials and Regulatory Approvals
Before any regenerative therapy can reach patients, it must undergo rigorous clinical trials to prove its safety and efficacy. This multi-phase process can take years, even decades, and requires immense financial investment and patient participation. For bioprinted organs and complex cell therapies, the regulatory path is particularly intricate because these are living products, not static drugs or devices. Regulators are grappling with how to assess long-term integration, potential for unintended growth, and immune responses. Despite these complexities, numerous clinical trials are currently underway globally, testing bioprinted skin grafts, cartilage repairs, pancreatic islets, and more. Each successful trial brings us one step closer to making these therapies a reality. The collaborative efforts between academic institutions, biotech companies, and regulatory agencies are crucial in streamlining this process, ensuring that promising treatments can move from research labs to the people who desperately need them as efficiently and safely as possible.
Closing Thoughts
As we’ve journeyed through the incredible landscape of bioprinting and stem cell research, it’s impossible not to feel a profound sense of optimism for the future of medicine. This isn’t just about incremental advancements; it’s about fundamentally reshaping how we approach health, disease, and the very concept of human longevity. The challenges ahead are significant, requiring immense dedication, ethical foresight, and continued collaboration across disciplines. Yet, the progress we’ve witnessed is a powerful testament to human ingenuity and our unwavering drive to heal. I genuinely believe we are on the cusp of a medical revolution that will deliver tailored, life-changing therapies to millions, making once-impossible treatments a tangible reality. Keep watching this space – the future of healthcare is being built, cell by cell.
Useful Information to Know
1. Bioprinting is more than just organs: While the dream of printing full organs captures headlines, much of the immediate impact is in creating 3D tissue models for drug testing, disease research, and personalized medicine, significantly reducing the need for animal trials.
2. Stem cells aren’t just from embryos: The breakthrough of induced pluripotent stem cells (iPSCs) means scientists can now reprogram adult cells (like skin cells!) back into a versatile, embryonic-like state, sidestepping many ethical concerns and enabling patient-specific therapies.
3. Bio-inks are complex: The “ink” used in bioprinting is a sophisticated mix of living cells, growth factors, and biocompatible materials. Its formulation is critical for ensuring cells survive, thrive, and form functional tissue after printing.
4. It’s a highly interdisciplinary field: Regenerative medicine is a melting pot of biology, engineering, material science, computer science, and clinical medicine. Progress relies heavily on experts from these diverse fields collaborating closely.
5. Ethical and regulatory discussions are ongoing: As these technologies advance, society is actively grappling with profound ethical questions (e.g., accessibility, “designer babies”) and working to establish robust regulatory frameworks to ensure safety and responsible innovation.
Key Takeaways
Bioprinting and stem cells are converging to create a new paradigm in healthcare, moving towards personalized, regenerative treatments. This revolution promises to address critical medical needs like organ shortages, accelerate drug discovery, and transform our approach to chronic diseases, despite facing significant ethical and regulatory hurdles.
Frequently Asked Questions (FAQ) 📖
Q: It all sounds incredibly futuristic, but when do you genuinely foresee bioprinted organs and tissues becoming a widespread reality for patients, and what’s the biggest hurdle standing in the way?
A: Oh, that’s the million-dollar question, isn’t it? And if I’m being completely honest, it’s not a simple ‘X years from now’ answer. From what I’ve seen and the conversations I’ve had with folks deeply embedded in this field, we’re definitely past the ‘proof of concept’ stage for simpler tissues.
Think cartilage, skin grafts, maybe even some vascular structures – those are already seeing limited clinical trials or specialized applications. I recall reading about a patient in Texas who received a 3D-bioprinted bone graft, and it just made my jaw drop.
But for complex, fully functional organs like a heart or a kidney? That’s a different beast entirely. We’re talking about integrating vast networks of blood vessels, nerves, and multiple cell types, ensuring they function perfectly for decades within a living body without rejection.
The major hurdle, in my opinion, isn’t just the printing technology itself, which is advancing at light speed, but rather the vascularization – getting tiny, intricate blood vessels to form and integrate properly to keep the cells alive and nourished within a large structure.
It’s like trying to build a city without a plumbing or electrical grid; everything just dies. We’re also grappling with the sheer scale-up for manufacturing and navigating the rigorous regulatory pathways of agencies like the FDA.
It’s a marathon, not a sprint, but every year brings us closer. My gut feeling? Widespread, complex organ bioprinting for the general public might still be a decade or two out, but for more specific, less complex tissue needs, we’re already seeing the dawn.
Q: This technology is so powerful it almost feels… unfathomable. What are some of the major ethical and safety concerns we need to be really grappling with as we advance in creating human tissues and organs?
A: You hit on a really crucial point there, and it’s something that keeps a lot of experts up at night, myself included. When you’re talking about essentially growing human body parts, the ethical landscape gets incredibly complex, incredibly fast.
On the safety front, the primary concern is always, always rejection and function. Will these new tissues or organs integrate seamlessly without causing an adverse immune response?
Will they perform their intended function for the long haul without unforeseen complications? What happens if the cells, once implanted, behave in an unexpected way?
We’re talking about living material, not a piece of plastic. Then there’s the ‘playing God’ narrative, which, while perhaps a bit dramatic for some, touches on a very real discomfort about manipulating life at such a fundamental level.
Beyond that, the big one for me is equitable access. If these treatments are groundbreakingly expensive at first, who gets them? Will it widen the health disparity gap, creating a two-tiered system where only the wealthy can afford a ‘new’ liver or heart?
That’s a societal question we have to address head-on, not just a scientific one. There are also questions around sourcing stem cells, especially embryonic ones, though induced pluripotent stem cells (iPSCs) are helping to mitigate some of those specific debates.
It’s a field where the science must walk hand-in-hand with robust ethical frameworks and public discourse, or we risk stumbling.
Q: Beyond the obvious, like growing a new kidney or heart, what are some of the truly revolutionary, perhaps less-discussed, applications of bioprinting and stem cell research that have you personally most excited?
A: Ah, now we’re talking about the truly mind-blowing stuff that gets me genuinely buzzed! While organ replacement is obviously life-saving, the applications extend far, far beyond that, and honestly, they’re changing how we think about disease and drug development.
One area that excites me immensely is personalized drug testing platforms. Imagine taking a patient’s own cells, bioprinting a tiny, functional mini-organ – a ‘liver-on-a-chip’ or ‘heart-on-a-chip’ – and then testing different drug compounds directly on their tissue before ever giving it to them.
This isn’t theoretical; companies are doing this right now! It could revolutionize how pharmaceuticals are developed, drastically reduce the need for animal testing, and crucially, minimize adverse drug reactions in patients.
Think of the cost savings and the lives saved from unexpected side effects! Another incredibly powerful application is creating complex disease models outside the human body.
Instead of just studying cells in a petri dish, researchers are now bioprinting structures that mimic cancerous tumors, neurological pathways affected by Alzheimer’s, or even sections of a diseased gut.
It allows for unprecedented, highly controlled observation of disease progression and therapeutic intervention without having to directly experiment on a living person.
It’s like having a microscopic, living sandbox where you can try things you never could before. When I first heard about a ‘brain organoid’ being used to study neurodegenerative diseases, I honestly got goosebumps.
These aren’t just academic curiosities; they’re vital tools pushing the boundaries of what we understand about human biology and pathology. It’s a game-changer, plain and simple.
📚 References
Wikipedia Encyclopedia
구글 검색 결과
구글 검색 결과
구글 검색 결과
구글 검색 결과
구글 검색 결과